Oxidation Number of K
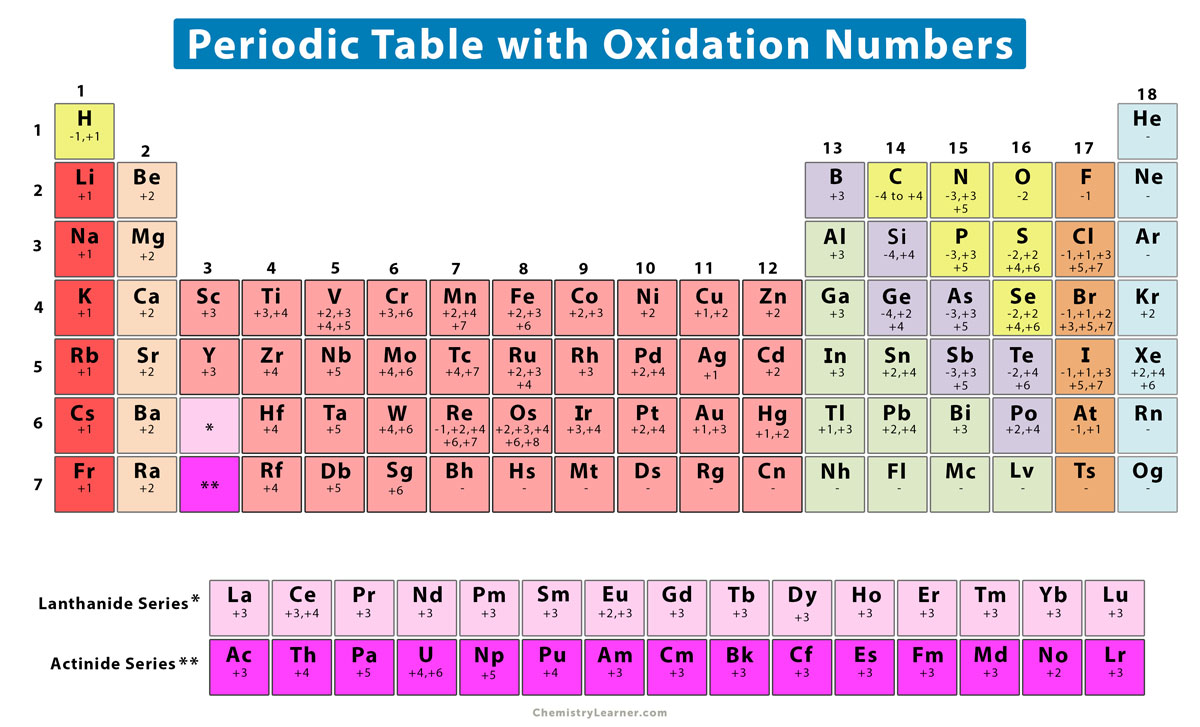
Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. 60 rows Monoatomic Ions Oxidation Numbers.
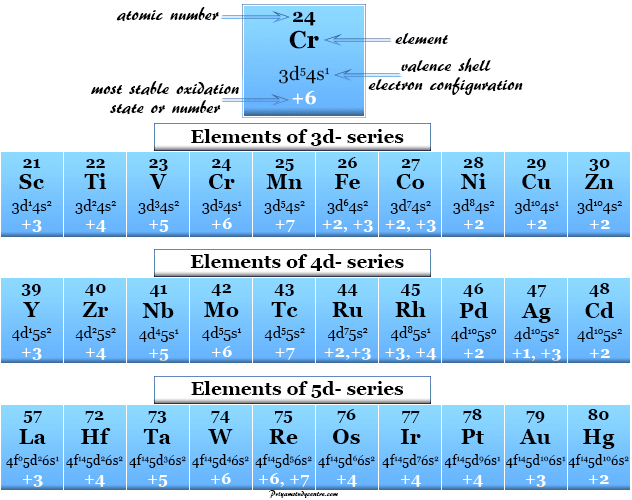
Oxidation Number Periodic Table Elements Definition Rules
Answer 1 of 3.
. However in the given question of finding out the oxidation number of cobalt in KCoCO 4 it is necessary to find out the oxidation state of potassium as well as that of carbon monoxide. To calculate oxidation numbers of elements in the chemical compound enter its formula and click Calculate for example. The common oxidation number.
Answer 1 of 7. 1 4 -2 x 0. In sodium compounds sodium only forms 1 oxidation number.
So let the oxidation state of K be x. Assign oxidation numbers to each element in all the compounds or ions. Therefore 1 2x 0.
Oxidation number of potassium. Name Symbol Oxidation number. Calculation of Oxidation Number of an Atom in a MoleculeIon.
According to rule 4 the. In K O 2 lets consider that the oxidation number of oxygen is x. X 7 The Oxidation.
Generally the Oxidation number is the. It has only one electron in its valence shell. Oxidation state of oxygen is -2.
But there are two Ks so 12 is 2. Oxidation number of K 4 oxidation number of oxygen oxidation number of Mn 0. The total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom is known as the Oxidation Number.
But some types of atoms such as chlorine form various oxidation numbers like -1 0 1 3 5 7 oxidation numbers in. To find the correct oxidation state of in K Potassium we use a few rules and some simple math. Consider the oxidation state of Potassium K x.
What is the oxidation number of K in KMnO. Free atoms H2 usually have an. The oxidation number of each K is 1.
Assigning these numbers involves several rules. Calculating the oxidation number of Potassium K in KO 2. In k2O2 oxygen is acting as a peroxidethe Oxidation state of peroxide is -1 2x -1 0 2x1 x -12 hence oxidation state of k in k2O2 is -1 hope you like it please upvote.
Lets take oxidation number of Manganese atom as x. Since we know that K 1 and K O 2 is a neutral molecule. What is the common oxidation number for inert gases.
In the case of superoxide the oxidation state of Oxygen O - 1 2. The oxidation number of rmO in rmKrmO_2 frac12 Q5. What is the oxidation number of K in KMnO4 OR K Mn O4.
Ca2 HF2- Fe4 Fe CN63 NH4NO3 so42- ch3cooh. Solved Examples on Oxidation Numbers. In addition to elemental Potassium K well also look at.
Oxidation number of oxygen in oxide ionO 2- is -2 and in peroxide ionO-O 2- is -1. 2x -2 0 overall oxidation state of a compound without charge is 0 x 1 Therefore oxidation state.

Group 15 Elements Oxidation State And Trends In Chemical Properties

Oxidation Number State Definition Rules How To Find And Examples
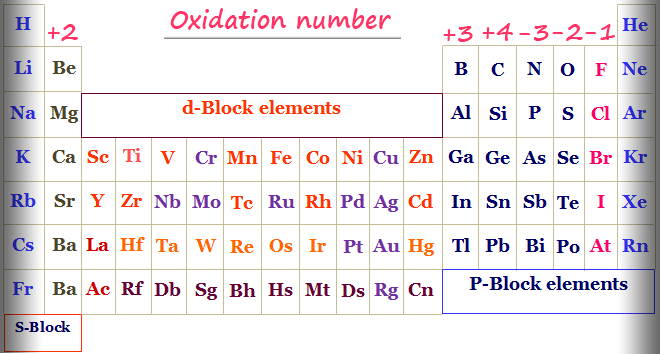
Oxidation Number Periodic Table Elements Definition Rules

0 Response to "Oxidation Number of K"
Post a Comment